Table of Contents
- Defining the Basics of Cellular Therapy
- Significant Advancements in Regenerative Medicine
- The Power of Immunotherapy: Including CAR T Cell Therapy and NK Cell Immunotherapy
- Navigating Clinical Trials and Gene Editing
- Emerging Trends in Biotechnology and Healthcare
Unlocking the Potential: How Cellular Therapy is Reshaping Modern Medicine
The world of Regenerative Medicine is rapidly experiencing a significant transformation, primarily powered by advances in Cellular Therapy. This approach leverages viable cells to regenerate faulty tissues and body parts. Biotechnology serves a crucial role in this process, providing the tools needed to manipulate these cells efficiently. Dissimilar to conventional pharmaceuticals, Cellular Therapy provides the possibility for permanent solutions instead of merely alleviating symptoms. We're seeing extraordinary advancements in this area. The convergence of biology and technology is opening new avenues for treating intractable conditions. Comprehending these principles is key for realizing the scale of this medical revolution.
Exploring the Spectrum: Specific Types of Advanced Cellular Therapy
Several pioneering treatments have come to the forefront under the umbrella of Cellular Therapy and Immunotherapy. These address illnesses on a cellular basis, providing new hope to sufferers. Among the most prominent examples are:
- CAR T Cell Therapy: A revolutionary method where a patient's T-cells are engineered to target malignancies.
- MSC Stem Cell Therapy: Leveraging multipotent stem cells for their potent regenerative and immune-modulating properties.
- NK Cell Immunotherapy: Activating Natural Killer (NK) cells to seek out and eliminate infected cells.
- Cytotoxic T Lymphocytes Therapy: Administering specific T-cells that can potently kill tumors.
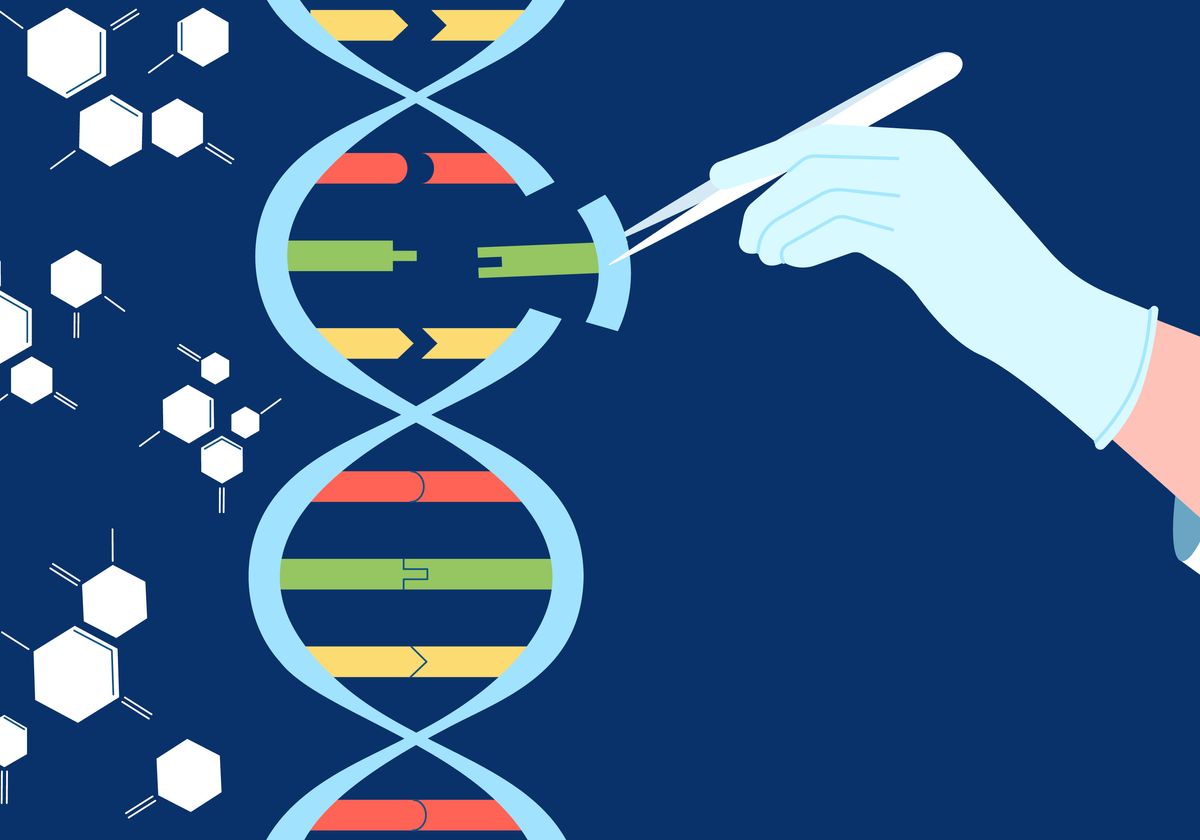
- Modern Gene Editing: Techniques such as CRISPR that accurately alter genetic material to cure hereditary disorders.
Every one of these approaches represents a significant leap ahead in Biotechnology. The progress is linked with ongoing research.
"We are poised at the very brink of a medical transformation. The incredible advancement in Regenerative Medicine and Immunotherapy isn't just gradual; it's truly game-changing, offering tangible possibilities for healing we once believed unachievable."
The Critical Pathway to Approval: The Importance of Clinical Trials and Gene Editing
The path from a novel Biotechnology concept to a approved therapy hinges on thorough Clinical Trials. These essential investigations are necessary to determine the security and effectiveness of emerging interventions like CAR T Cell Therapy. At the same time, breakthroughs in Gene Editing are becoming more and more central to the field. This powerful tool enables researchers to repair genetic mutations at their actual origin. The responsible use of Gene Editing in the context of Clinical Trials is currently paving the way for personalized medicine. Absent this meticulous approval process, even the advanced MSC Stem Cell Therapy or NK Cell Immunotherapy cannot responsibly benefit the general public. Therefore, ongoing funding in both fields is critically vital for upcoming medical progress.
Understanding Different Therapy Modalities
| Therapy Characteristic | CAR T Cell Therapy | MSC Stem Cell Therapy | NK Cell Immunotherapy |
|---|---|---|---|
| Primary Origin | Autologous Immune Cells | Donated Umbilical Cord/Marrow | Patient or Donor NK Cells |
| Primary Action | Genetically Modified Targeting of Cancer | Healing & Immune Modulation | Innate Tumor-Killing Response |
| Primary Application | Leukemia/Lymphoma | Degenerative Diseases | Various Malignancies (including Liquid) |
"Joining the Clinical Trials for MSC Stem Cell Therapy was profound decision for my me. Following years of struggling with a chronic condition, this Regenerative Medicine treatment offered me tangible relief. The professionals was incredibly caring throughout the whole journey. I truly feel this is the dawning of healthcare. I am so thankful for the opportunity."
– Jennifer K.
"My father's fight with lymphoma led us to the cutting-edge field of Immunotherapy. The doctors suggested CAR T Cell Therapy when conventional options had unfortunately stopped working. The Biotechnology involved in it is truly astounding. Seeing his body's own cells engineered to fight the disease was like a miracle. We're now observing positive results, all because of this type of Cellular Therapy."
– Michael R.
Patient Success Stories
"I coping with a chronic autoimmune disorder for more than a decade. Conventional protocols provided only minimal help and came with harsh complications. My eventually recommended the idea of Regenerative Medicine, specifically MSC Stem Cell Therapy. I initially apprehensive, yet the was promising. I decided to proceed and the outcomes have been nothing short of astounding. It was not instant, but over months, my decreased, my energy improved, and my overall quality of daily life has improved. This Cellular Therapy didn't just mask my; it felt like it's addressing the root cause. The in Biotechnology are genuinely giving individuals like me their futures again. I'm eternally grateful to the pioneers advancing this incredible science forward."
Commonly Asked Questions regarding Cellular Therapy and Immunotherapy
- Q: What is the difference between Cellular Therapy and Immunotherapy?
A: While they are very linked, Immunotherapy is a broad category for treatments that leverage the body's own defenses to attack illness (e.g., cancer). Cellular Therapy is a specific type of treatment which uses administering living cells into a patient to elicit a healing effect. Many treatments, such as CAR T Cell Therapy, are actually both. - Q: Are MSC Stem Cell Therapy or Gene Editing fully approved?
A: The safety profile of these treatments is a primary concern of all ongoing Clinical Trials. While MSC Stem Cell Therapy has a robust safety record profile for specific applications, Gene Editing is a emerging technology with safety guidelines and oversight. Approval varies significantly by country and the specific disease that is treated. - Q: How does Cytotoxic T Lymphocytes Therapy differ from NK Cell Immunotherapy?
A: Both therapies use immune effector cells, but are part of distinct branches of the immune system response. CTLs are part of the specific immune CAR T Cell Therapy system response, which means they must to be 'trained' or activated to a particular target (e.g., a virus-infected cell). NK Cell Immunotherapy are a component of the inbuilt immune system response, enabling them detect and kill abnormal cells more broadly without prior activation.